Pfizer Submitting Data for Vaccine Use in Kids
by Yusra Husain ’22
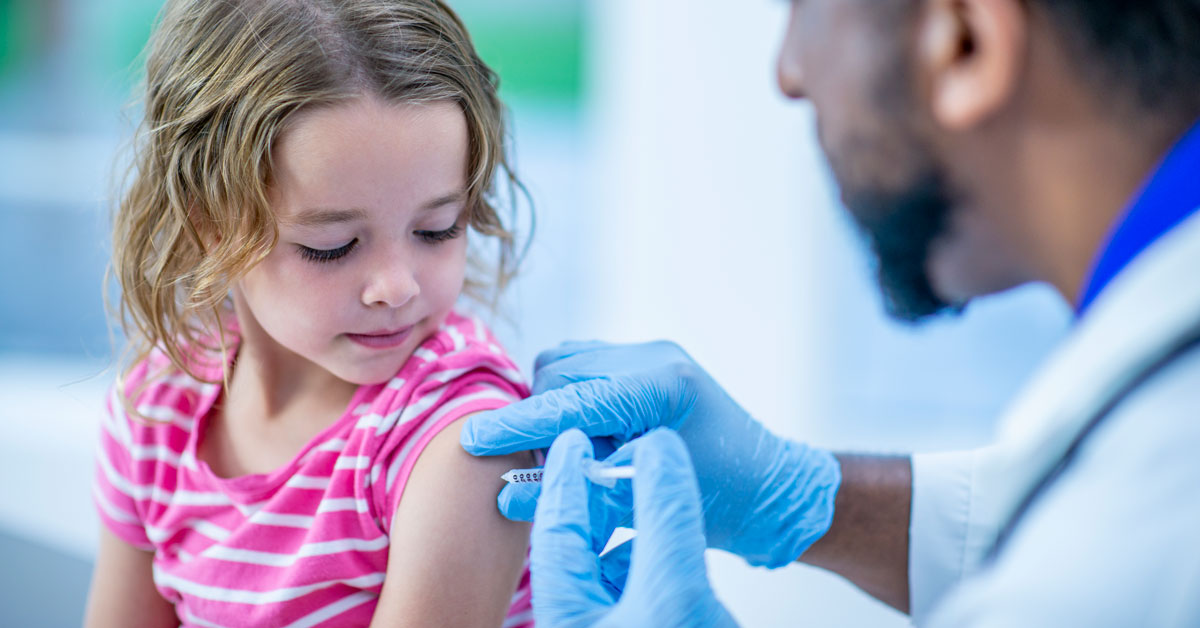
 The United States is finally a step closer toward higher vaccination rates for all school-aged children; Pfizer and its German partner, BioNTech, recently announced that their Covid-19 vaccine is now safe for ages 5-11 as it promotes a stronger immune response in younger children. This news comes after a recent rise in pediatric Covid-19 cases. Since July, pediatric cases have increased by 240 percent, and as of now, 26 percent of nationwide cases are reported in children. With the overall rate increasing in youth populations, Pfizer has provided a light at the end of the tunnel for many families with young children.
The United States is finally a step closer toward higher vaccination rates for all school-aged children; Pfizer and its German partner, BioNTech, recently announced that their Covid-19 vaccine is now safe for ages 5-11 as it promotes a stronger immune response in younger children. This news comes after a recent rise in pediatric Covid-19 cases. Since July, pediatric cases have increased by 240 percent, and as of now, 26 percent of nationwide cases are reported in children. With the overall rate increasing in youth populations, Pfizer has provided a light at the end of the tunnel for many families with young children.
This news is the result of Pfizer’s successful and highly anticipated trial, which studied the effectiveness and safety of vaccinations for children. Although the initial phases of the trial began in June, the process had been pushed back slightly due to the Food and Drug Administration’s (FDA) request for the enrollment of more children to make the results stronger. The trial eventually included 2,268 participants ranging between the ages of 5 and less than 12.
Since then, the results demonstrate that the side effects are comparable to those of individuals between the ages of 16-25. The trial utilized a smaller dosage – 10 micro grams instead of 30 micro grams – selected for the safety of children on a two-dose schedule. This dosage produced similar and in some cases fewer side effects, such as sore arms, achiness, or fevers.
Pfizer now seeks approval for this vaccine, and has planned to submit data to an abundance of regulatory agencies prior to the start of winter. As of September 28, data has been submitted to the FDA for initial review. Ultimately, the Center for Disease Control (CDC) and the FDA will have the final say for the vaccine’s administration. Unfortunately, the roll-out may be difficult as booster shots are currently being administered as well, but CDC director Dr. Rochelle Walensky stated that this vaccine will be reviewed with urgency. The public is eager to hear back, and results are expected soon. We can also anticipate information regarding vaccines for children under the age of 5 later in the year.